Follow our Aurevia LinkedIn channels
Visit us on LinkedIn to stay informed about industry news, events, and guidelines.
Connect with us
Whether you're a manufacturer, innovator, or healthcare professional, visit our LinkedIn channels to get expert tips and strategies to support your journey.
Aurevia Clinical Research Services CRO
Get insights into clinical research and advancing patient outcomes.
Aurevia Medtech Compliance
Get regulatory updates and insights into best practices in compliance and quality.
Latest news

Notice about a change in some of our email addresses
As part of the upcoming company split on 1 January 2026, some of our email domains will change to @labquality.com...

How do you shape regulatory complexity into strategic clarity?
As AI continues to reshape healthcare, clear guidance on health data use is more critical than ever. Building on the...

Fast track for mononational clinical trial applications in Sweden
Starting September 1, 2025, the Swedish Medical Products Agency (Läkemedelsverket) introduced a fast-track process for...

Upcoming webinar: what IVDR Article 5(5) means for your lab
Whether your lab is already working under IVDR and Article 5(5), evaluating tests, or preparing for transition, this...
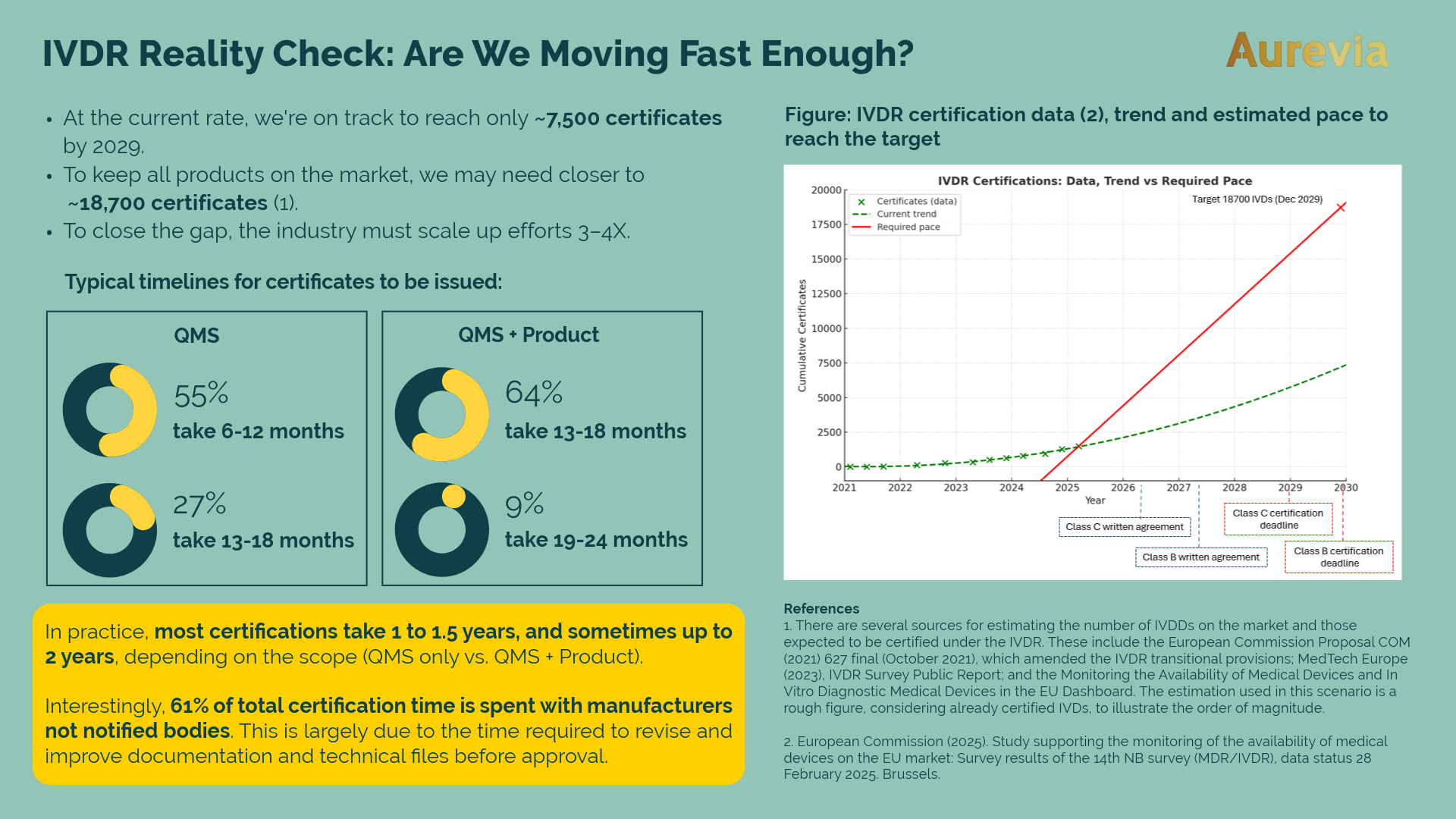
IVDR reality check: are we moving fast enough?
As of February 2025, approximately 1,500 IVDR certificates have been issued. That’s progress — but is it enough? With...