Fast track for mononational clinical trial applications in Sweden
Starting September 1, 2025, the Swedish Medical Products Agency (Läkemedelsverket) introduced a...
Here are the latest news from Aurevia. Keep track of what's happening!

Starting September 1, 2025, the Swedish Medical Products Agency (Läkemedelsverket) introduced a...
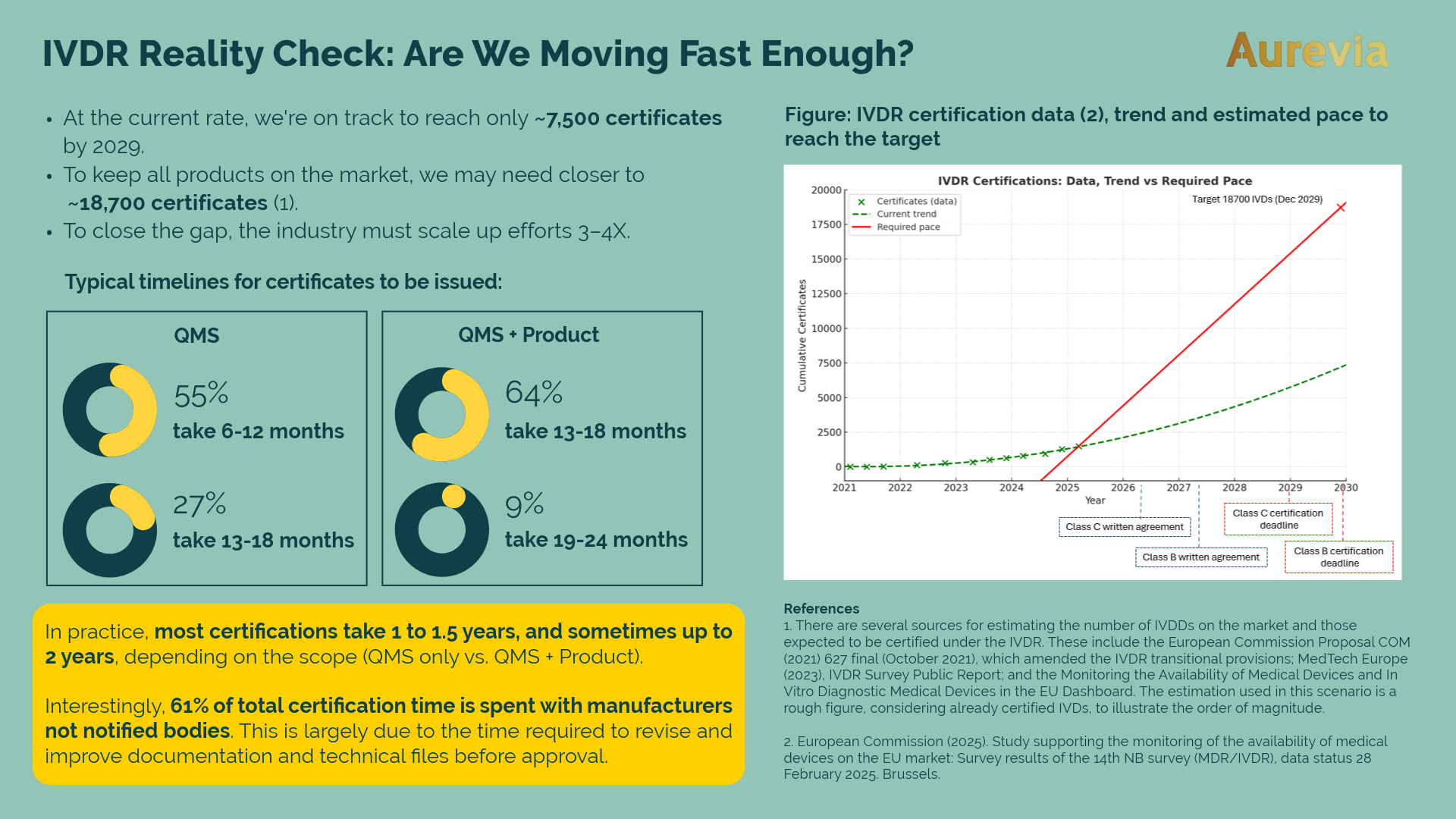
As of February 2025, approximately 1,500 IVDR certificates have been issued. That’s progress — but...

Although the above statement is intentionally somewhat provocative, it does hold a degree of truth....

FDA’s final guidance on Computer Software Assurance (CSA) for Production and Quality System...

In an industry shaped by rapid regulatory changes and global competition, leading life science...

As transparency and consistency become increasingly emphasized by the FDA, medical device...

In the second part of our article series on the FDA’s new “radical transparency” initiative, we...

In a significant policy shift, the FDA has begun publishing Complete Response Letters (CRLs) for...

ISO 10993-1, the core standard for the biological evaluation of medical devices, has been under...

Post-Market Surveillance (PMS) is more than complaint handling – it’s a strategic tool for patient...
In the competitive and highly regulated world of medical technology, a robust and well-structured...

The industry's financing climate has slowed down considerably. Investments in the development of...