Welcome to celebrate the 50th anniversary of the Labquality Days Congress!
In 2026, Labquality Days turns 50, and we want to celebrate this milestone together with you.
Here are the latest news from Aurevia. Keep track of what's happening!

In 2026, Labquality Days turns 50, and we want to celebrate this milestone together with you.

As we wrap up an incredible year, we want to express our heartfelt gratitude to our amazing...

Please note that as part of the upcoming company split on 1 January 2026, some of our email domains...

As AI continues to reshape healthcare, clear guidance on health data use is more critical than...

Starting September 1, 2025, the Swedish Medical Products Agency (Läkemedelsverket) introduced a...

The EU In Vitro Diagnostic Regulation (IVDR) is transforming how clinical laboratories develop,...
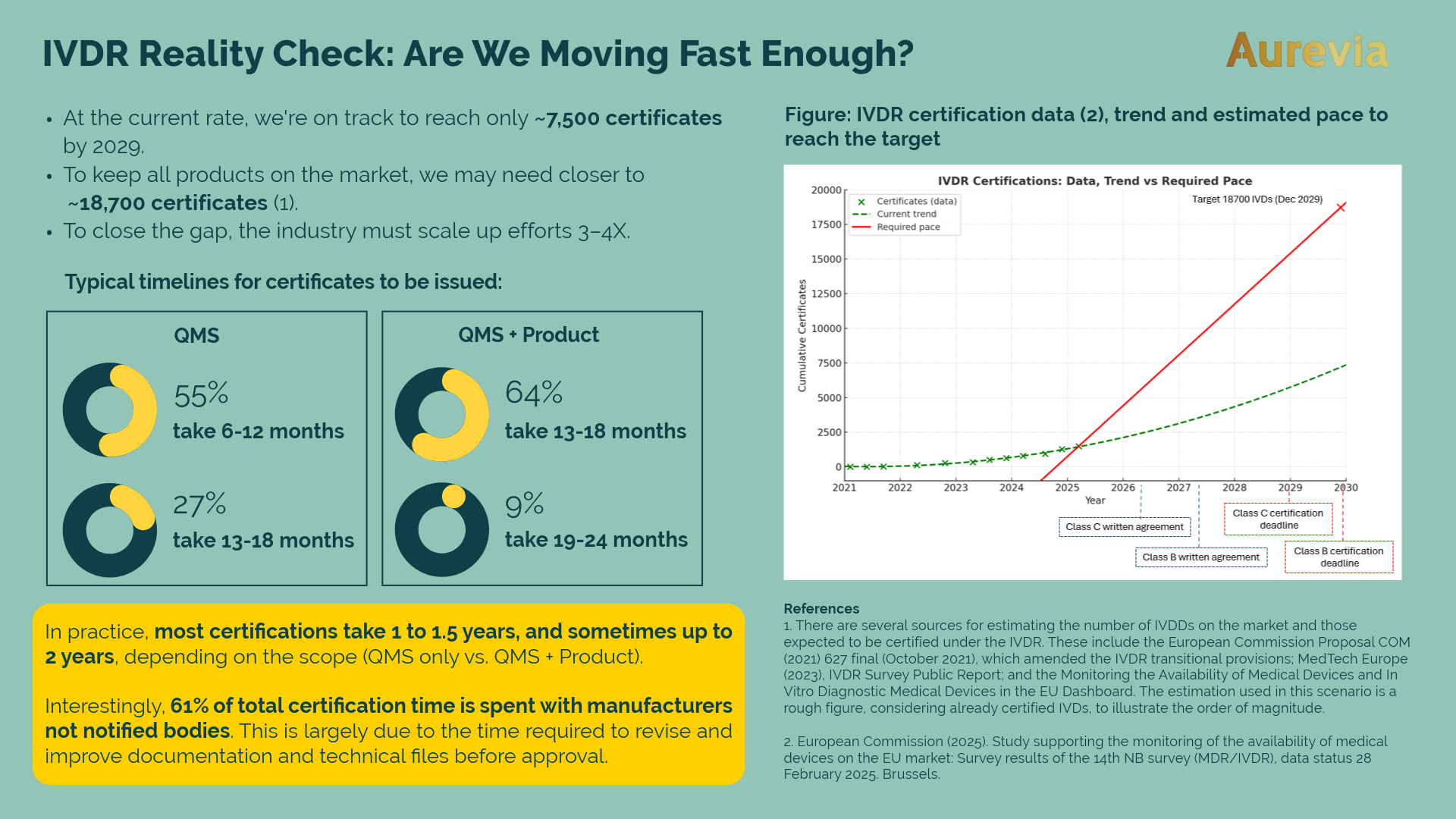
As of February 2025, approximately 1,500 IVDR certificates have been issued. That’s progress — but...

Although the above statement is intentionally somewhat provocative, it does hold a degree of truth....

FDA’s final guidance on Computer Software Assurance (CSA) for Production and Quality System...

In an industry shaped by rapid regulatory changes and global competition, leading life science...

As transparency and consistency become increasingly emphasized by the FDA, medical device...

In the second part of our article series on the FDA’s new “radical transparency” initiative, we...