QUALITY ASSURANCE
US FDA QSR
Our expertise with quality systems, including the USA Food and Drug Administration Quality System Regulation, QSR, 21 CFR Title 820 supports your medical device through trials.
Quality System Regulation 21 CFR 820
According to the regulation, it is mandatory for manufacturers to:
- Establish that the quality system is consistent with the complexity of the device, manufacturing processes and size, and the complexity of the manufacturing facility
- Plan to define and implement effective procedures
- Implement what has been documented and is going to be done
- Check the system and make necessary changes (CAPA)
- Act upon changes and ensure they are implemented
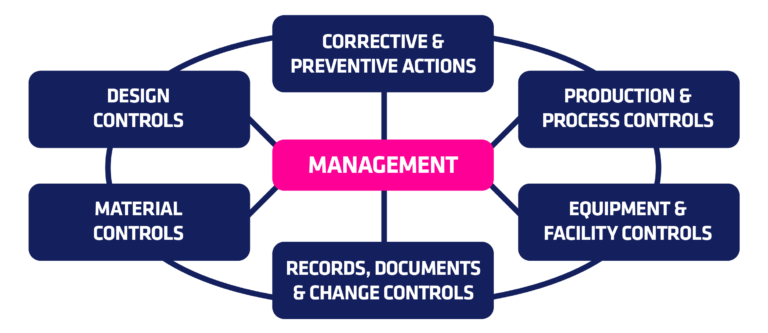
Quality System Regulation 21 CFR 820
According to the regulation, it is mandatory for manufacturers to:
- Establish that the quality system is consistent with the complexity of the device, manufacturing processes and size, and the complexity of the manufacturing facility
- Plan to define and implement effective procedures
- Implement what has been documented and is going to be done
- Check the system and make necessary changes (CAPA)
- Act upon changes and ensure they are implemented
How can we help?
We can assist you with
- Planning the QMS together with the manufacturer according to the QSR regulation
- Identifying all processes needed for their QSR
- Preparing the Quality Manual, QMS process descriptions, templates, databases, and other documentation needed together with the manufacturer
- Choosing and implementing electronic QMS when needed
- Maintaining and continually improving your QMS
- Daily QMS activities including nonconformances, feedback, complaints, internal and supplier audits, vigilance, and many others
- Offering customized and open training for medical device QMS
Explore our services

Quality Management System ISO 13485
Setting up quality management systems (QMS) for medical device manufacturers.

US FDA 21 CFR Title 820 (QSR)
We know quality systems, including the USA Food and Drug Administration Quality System Regulation, QSR, 21 CFR Title 820.

QMS Improvement and Gap Analysis
Improvement of medical device manufacturer’s quality management system (QMS) and GAP analysis.
Latest news
Read the latest news from the world of quality.

Notice about a change in some of our email addresses
As part of the upcoming company split on 1 January 2026, some of our email domains will change to @labquality.com...

How do you shape regulatory complexity into strategic clarity?
As AI continues to reshape healthcare, clear guidance on health data use is more critical than ever. Building on the...

Fast track for mononational clinical trial applications in Sweden
Starting September 1, 2025, the Swedish Medical Products Agency (Läkemedelsverket) introduced a fast-track process for...

Upcoming webinar: what IVDR Article 5(5) means for your lab
Whether your lab is already working under IVDR and Article 5(5), evaluating tests, or preparing for transition, this...
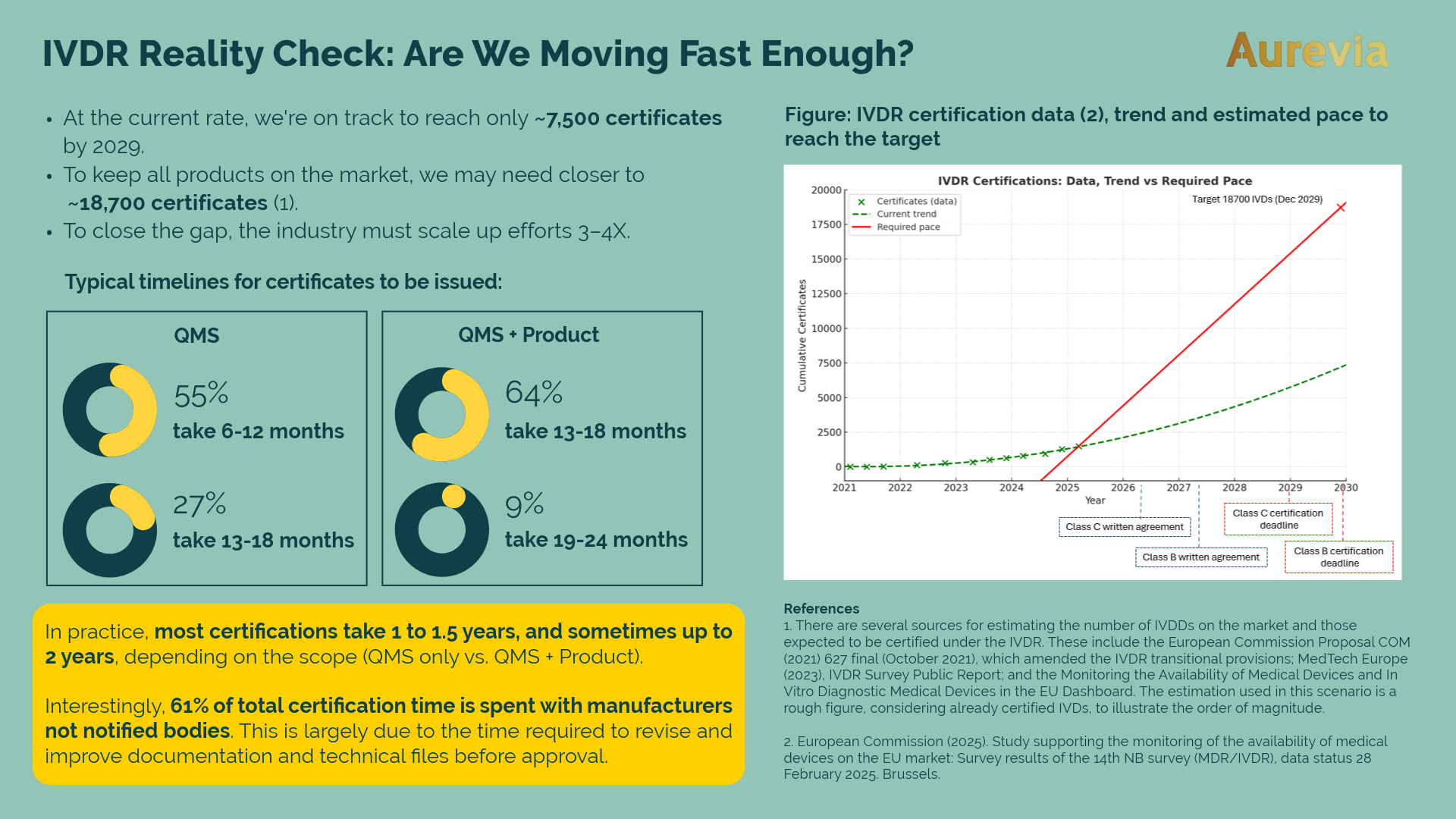
IVDR reality check: are we moving fast enough?
As of February 2025, approximately 1,500 IVDR certificates have been issued. That’s progress — but is it enough? With...